News
Events

USP 4 Flow-Through Cell: The Ideal Tool for Dissolution Testing of Novel Drug Delivery Systems Introduction In the pharmaceutical industry, USP 4 flow-through cell dissolution testing is essential for ensuring that drugs work as intended and meet quality...
Automated Dissolution Tester Apparatus: Fast, Accurate & 21 CFR Part 11 Compliance
Introduction Dissolution testing ensures that tablets and capsules release their APIs at the intended rate. It's crucial for predicting drug performance, regulatory submissions, and batch consistency. As formulations become complex and data integrity...

Dissolution to permeation to bio-availability: Connecting the dots!

Development of Biphasic Dissolution model for vaginal tablets containing BCS class-II drug

Significance of electromagnetic sieve shaker

Reducing errors and increasing throughput in Content Uniformity & Assay Testing.

Reducing errors and increasing throughput in Content Uniformity & Assay Testing.

In-vitro drug release methods for liposomal drug delivery system
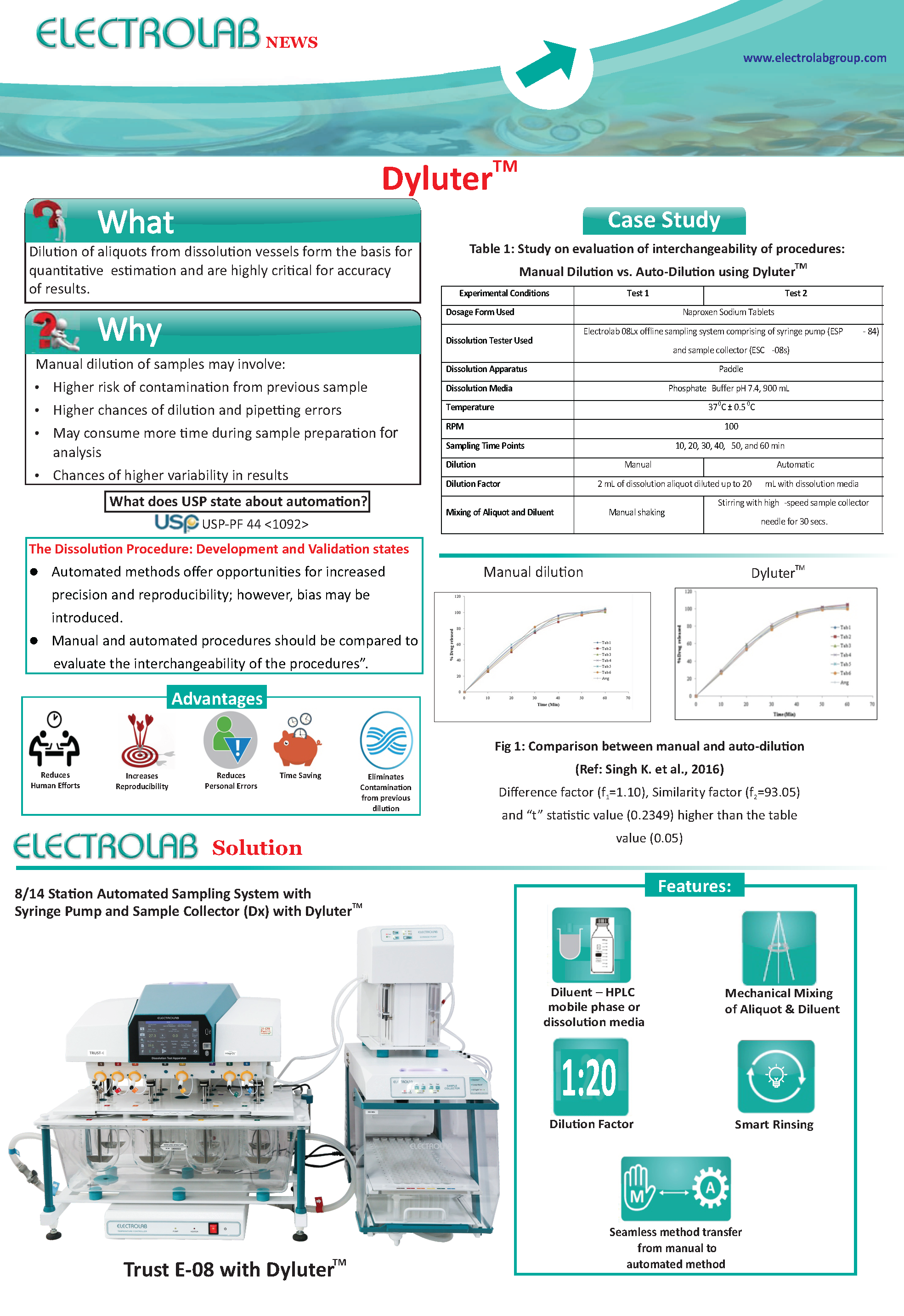
Dyluter: Auto-Dilution and Auto-Mixing of dissolution aliquotes